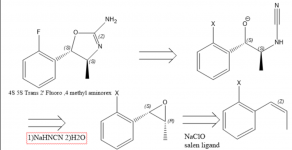
There is prevailing interest in 4 Methylaminorex and its derivatives, in part owning to it's scarcity. Pharmacological study's suggest that the rank order of potencies for 4 Methylaminorex enatiomers is trans-4S,5S > cis-4S,5R ~ cis-4R,5S > trans-4R,5R and racemic Trans methylaminorex is considered more potent than cis (1), Therefore a directed synthesis toward the Trans 4S, 5S enatiomer is very desirable. In 1970 a patent is published relating to a synthesis of Aminorex from styrene oxide with the use of cyanamide(2), which can allude to the fact that an enatiopure styrene oxide or beta methyl styrene/ propenyl benzene oxide would lead to the requisite Methylaminorex isomer (2). In order to obtain the 4S,5S derivative, the 1S,2R propenyl benzene epoxide must be used because the stereochemistry at that chiral center is inverted. The epoxide is easily obtainable via Jacobsen epoxidation(3) however I am unsure of the best way of obtaining substituted propenyl benzene's concisely.

Secondly, it has it is clear that a new possibly psychoactive derivative of the 2 amino oxazoline class is possible as is seen with indane derivatives of amphetamine such as methylenedioxy 2 amino indane (MDAI) and hence it would follow that the cyclic equivalent of norephedrine, cis 2 amino 1 indanol would, on treatment with cyanogen bromide also yield 2 amino oxazolines(4). In the example provide below the stereochemistry of the indane is 1R,2S as it is expected to be more potent than the enatiomer. The synthesis of this compound would best be achieved by an intra molecular friedel crafts acylation of anti/threo 1R,2R beta hydroxy phenylalanine (5). However, there are difficulties in making this compound, it could be achieved by an aminohydroxylation of a cinnamate followed by mitsunobu inversion (6) or by oxidation of a beta bromo phenylalanine with silver nitrate (7).


(1) https://web.archive.org/web/2007093...am/journal_v3_num34/journal_v3_num34_pg6.html
(2) https://patents.google.com/patent/DE2101424A1/en?oq=DE+Patent+2101424 (b) examples of use of sodium hydrogen cyanamide for prep of 2 amino oxazolines; (a) http://tch.ucsd.edu/pdfs/EJOC_7337_2013.pdf
(3) E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker, L. Deng, J. Am. Chem. Soc., 1991, 113, 7063-7064
(4) (a) https://sci-hub.hkvisa.net/https://doi.org/10.1021/jm00339a011 / https://pubs.acs.org/doi/10.1021/jm00339a011 (b) https://www.erowid.org/archive/rhodium/chemistry/4-mar.stereoisomers.html
(5) (a) http://koreascience.or.kr/article/JAKO200602727234043.pdf
(6) (a) https://sci-hub.hkvisa.net/https://doi.org/10.1021/jo010482c, (b) https://sci-hub.hkvisa.net/10.1039/C3RA44631J
(7) https://sci-hub.hkvisa.net/10.1021/jo061159i
Secondly, it has it is clear that a new possibly psychoactive derivative of the 2 amino oxazoline class is possible as is seen with indane derivatives of amphetamine such as methylenedioxy 2 amino indane (MDAI) and hence it would follow that the cyclic equivalent of norephedrine, cis 2 amino 1 indanol would, on treatment with cyanogen bromide also yield 2 amino oxazolines(4). In the example provide below the stereochemistry of the indane is 1R,2S as it is expected to be more potent than the enatiomer. The synthesis of this compound would best be achieved by an intra molecular friedel crafts acylation of anti/threo 1R,2R beta hydroxy phenylalanine (5). However, there are difficulties in making this compound, it could be achieved by an aminohydroxylation of a cinnamate followed by mitsunobu inversion (6) or by oxidation of a beta bromo phenylalanine with silver nitrate (7).
(1) https://web.archive.org/web/2007093...am/journal_v3_num34/journal_v3_num34_pg6.html
(2) https://patents.google.com/patent/DE2101424A1/en?oq=DE+Patent+2101424 (b) examples of use of sodium hydrogen cyanamide for prep of 2 amino oxazolines; (a) http://tch.ucsd.edu/pdfs/EJOC_7337_2013.pdf
(3) E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker, L. Deng, J. Am. Chem. Soc., 1991, 113, 7063-7064
(4) (a) https://sci-hub.hkvisa.net/https://doi.org/10.1021/jm00339a011 / https://pubs.acs.org/doi/10.1021/jm00339a011 (b) https://www.erowid.org/archive/rhodium/chemistry/4-mar.stereoisomers.html
(5) (a) http://koreascience.or.kr/article/JAKO200602727234043.pdf
(6) (a) https://sci-hub.hkvisa.net/https://doi.org/10.1021/jo010482c, (b) https://sci-hub.hkvisa.net/10.1039/C3RA44631J
(7) https://sci-hub.hkvisa.net/10.1021/jo061159i
Attachments
Last edited by a moderator: