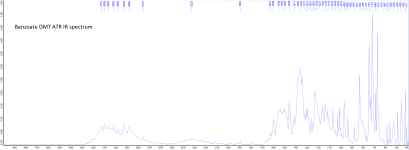
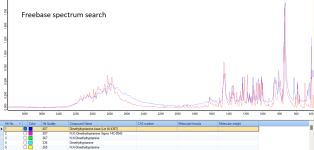
Tryptamine is converted to DMT in two steps. In the first step, three methyl groups are attached to the amine nitrogen. In the second step, one of them is removed.
1) Overmethylation
In a 100 mL Erlenmeyer flask suspend 4 g tryptamine in ~25 mL THF or MeCN. Use fast stirring with a long (40 mm) stir bar. Add 16-20 g methyl tosylate dissolved in additional ~25 mL of the same solvent.
There will be a temporary exothermic effect; precipitation of solids will begin almost immediately. Add 4 g fine and dry sodium (not potassium) carbonate powder in small portions. Any mass stuck to the walls of the flask should be broken up to ensure the reaction mixture is a homogeneous, runny, fast-swirling suspension - add a bit more solvent, if necessary.
You have two options: either leave the mixture stirring for several (e.g. three) days at room temperature, or raise the hotplate temperature to ~70 °C (~50 °C internal) and stir it for (up to) 12 hours.
Periodically scrape the walls of the flask down.
Refrigerate the reaction mixture and filter it under vacuum, washing with small amounts of cold dry acetone until the discarded filtrate is colorless. You can expect 13+ g (in MeCN) or 19+ g (in THF) white powder, presumably a mixture of N,N,N-trimethyltryptammonium tosylate (TMT tosylate), two equivalents of sodium tosylate, and some residual sodium carbonate.
Notes:
- You can monitor the evolution of CO2 with a gas bubbler connected to the flask.
- Since methyl tosylate is prone to alkaline hydrolysis, it may be a good idea to add sodium carbonate over several hours, each time after the CO2 evolution slows down.
- The yield is so suspiciously higher in THF compared to MeCN that one has to wonder if some unreacted tryptamine contaminates the product. There has been no evidence of that so far.
- To increase the yield you can boil some solvent (and water) off before filtering.
- Low yields at this step may indicate problems like degraded methyl tosylate (distill it under vacuum and store in a freezer) or excessive amount of solvents.
- These solvents are not recommended: NMP (high solubility of tosylates), methanol (tryptamine reacts with CO2 resulting in methyl carbamate - see the first attached paper), DMF (3% carbamate formation reported - see the second attached paper)
- These solvents will not work: DMSO (gets methylated itself into trimethyl sulfoxonium tosylate), ketones (imine formation), acetate esters (N-acetylation).
- The solubility of tosylates in acetone is unknown so it makes sense to wash with small amounts and keep the acetone cold and dry.
- The mix of salts can be recrystallized from hot water but the solubility of TMT tosylate in water relative to that of sodium tosylate is unknown.
2) Dequaternization
Suspend the mixture of salts obtained in the previous step in 25 mL ethanolamine in a 100 mL round-bottom flask flushed with argon and equipped with a thermometer and a gas bubbler. With intensive stirring, heat the suspension to 160-165 °C - its transparency will increase as the salts dissolve. Stir the reaction mixture for (up to) 5 hours. Use a fan to cool the flask down to room temperature.
Dilute the reaction mixture with 125 mL water basified with 2 g potassium carbonate. Extract DMT with N-amyl acetate (4 x 20 mL), wash the combined extracts 1-3 times with 5 mL NaCl brine and pour (filtering is optional) into a flask with excess (~5 g) benzoic acid. Stir the mixture to cause DMT benzoate to form and precipitate. Increase the temperature until all solids redissolve and the solution becomes fully transparent. Let DMT benzoate crystallize by slowly cooling the flask down to freezer temperatures. Filter the crystals, washing with heptane.
The post-crystallization yield of DMT benzoate should be over 6.1 g (~79% theoretical, methylated in THF over 12 hours). It can be recrystallized from hot amyl acetate - or, with greater losses, from hot water.
Notes:
- Let the inert gas slowly seep into the flask during the entire reaction (heating and cooling included) to maintain positive pressure and protect the hot amines from atmospheric oxygen.
- To avoid decomposition it is better to add a bit more crystallization solvent instead of heating the extract beyond 100 °C.
- Basic water with ethanolamine has a greenish tint under 365 nm UV light; amyl acetate is blue, high DMT content makes it fluoresce.
1) Overmethylation
R-NH2 + 3MeOTs + Na2CO3 🡒 R-NMe3.OTs + 2NaOTs + H2O + CO2🡑
In a 100 mL Erlenmeyer flask suspend 4 g tryptamine in ~25 mL THF or MeCN. Use fast stirring with a long (40 mm) stir bar. Add 16-20 g methyl tosylate dissolved in additional ~25 mL of the same solvent.
There will be a temporary exothermic effect; precipitation of solids will begin almost immediately. Add 4 g fine and dry sodium (not potassium) carbonate powder in small portions. Any mass stuck to the walls of the flask should be broken up to ensure the reaction mixture is a homogeneous, runny, fast-swirling suspension - add a bit more solvent, if necessary.
You have two options: either leave the mixture stirring for several (e.g. three) days at room temperature, or raise the hotplate temperature to ~70 °C (~50 °C internal) and stir it for (up to) 12 hours.
Periodically scrape the walls of the flask down.
Refrigerate the reaction mixture and filter it under vacuum, washing with small amounts of cold dry acetone until the discarded filtrate is colorless. You can expect 13+ g (in MeCN) or 19+ g (in THF) white powder, presumably a mixture of N,N,N-trimethyltryptammonium tosylate (TMT tosylate), two equivalents of sodium tosylate, and some residual sodium carbonate.
Notes:
- You can monitor the evolution of CO2 with a gas bubbler connected to the flask.
- Since methyl tosylate is prone to alkaline hydrolysis, it may be a good idea to add sodium carbonate over several hours, each time after the CO2 evolution slows down.
- The yield is so suspiciously higher in THF compared to MeCN that one has to wonder if some unreacted tryptamine contaminates the product. There has been no evidence of that so far.
- To increase the yield you can boil some solvent (and water) off before filtering.
- Low yields at this step may indicate problems like degraded methyl tosylate (distill it under vacuum and store in a freezer) or excessive amount of solvents.
- These solvents are not recommended: NMP (high solubility of tosylates), methanol (tryptamine reacts with CO2 resulting in methyl carbamate - see the first attached paper), DMF (3% carbamate formation reported - see the second attached paper)
- These solvents will not work: DMSO (gets methylated itself into trimethyl sulfoxonium tosylate), ketones (imine formation), acetate esters (N-acetylation).
- The solubility of tosylates in acetone is unknown so it makes sense to wash with small amounts and keep the acetone cold and dry.
- The mix of salts can be recrystallized from hot water but the solubility of TMT tosylate in water relative to that of sodium tosylate is unknown.
2) Dequaternization
R-NMe3.OTs + HOCH2CH2NH2 🡒 R-NMe2 + HOCH2CH2NHMeOTs
Suspend the mixture of salts obtained in the previous step in 25 mL ethanolamine in a 100 mL round-bottom flask flushed with argon and equipped with a thermometer and a gas bubbler. With intensive stirring, heat the suspension to 160-165 °C - its transparency will increase as the salts dissolve. Stir the reaction mixture for (up to) 5 hours. Use a fan to cool the flask down to room temperature.
Dilute the reaction mixture with 125 mL water basified with 2 g potassium carbonate. Extract DMT with N-amyl acetate (4 x 20 mL), wash the combined extracts 1-3 times with 5 mL NaCl brine and pour (filtering is optional) into a flask with excess (~5 g) benzoic acid. Stir the mixture to cause DMT benzoate to form and precipitate. Increase the temperature until all solids redissolve and the solution becomes fully transparent. Let DMT benzoate crystallize by slowly cooling the flask down to freezer temperatures. Filter the crystals, washing with heptane.
The post-crystallization yield of DMT benzoate should be over 6.1 g (~79% theoretical, methylated in THF over 12 hours). It can be recrystallized from hot amyl acetate - or, with greater losses, from hot water.
Notes:
- Let the inert gas slowly seep into the flask during the entire reaction (heating and cooling included) to maintain positive pressure and protect the hot amines from atmospheric oxygen.
- To avoid decomposition it is better to add a bit more crystallization solvent instead of heating the extract beyond 100 °C.
- Basic water with ethanolamine has a greenish tint under 365 nm UV light; amyl acetate is blue, high DMT content makes it fluoresce.